A DNA motor protein frequently encounters DNA binding proteins and complexes that can interfere, stall, or collapse essential processes. How these roadblocks are resolved or overcome can depend on multiple factors, including their location, the binding strength of interactions, and how they are removed/relocated.
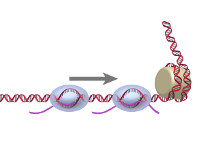 RNAP RNAPDuring transcription, RNA polymerase must access DNA, however nucleosomes can impose a substantial barrier to transcription elongation as they may induce RNAP pausing and arrest due to backtracking. We directly monitored RNAP and showed that histone-DNA interactions dictate RNAP pausing behavior and a trailing RNAP may reduce backtracking and enhance transcription of a leading RNAP. This alleviation of nucleosome-induced may serve as a mechanism for overcoming the nucleosomal barrier in vivo (Jin et al., NSMB, 2010). |
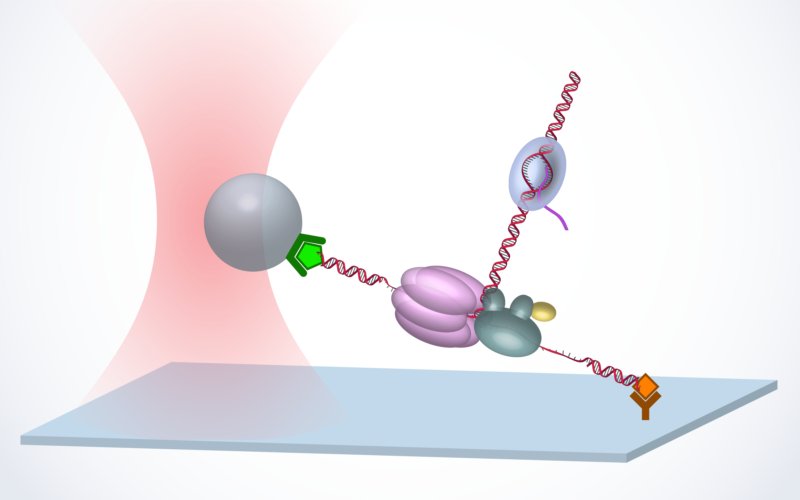
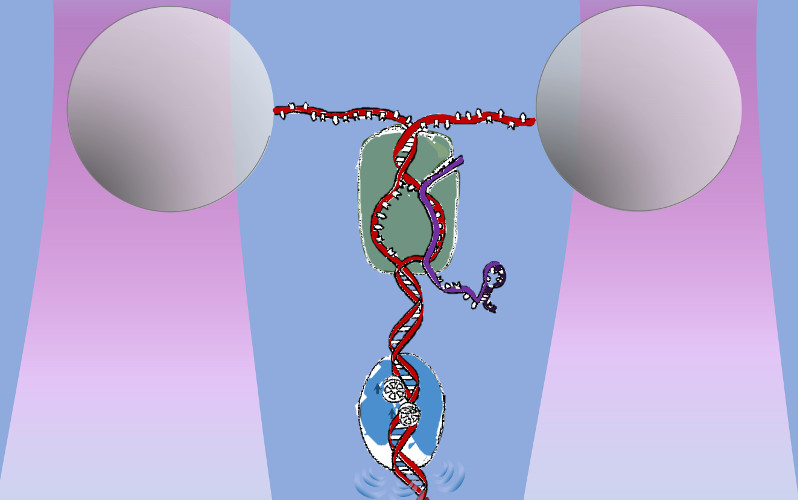 Mfd MfdRNAP may also be regulated by different motor proteins. E. coli Mfd targets a stalled RNAP and is best known for its role in bacterial transcription coupled repair. Using our a novel real‐time assay, termed an ‘unzipping tracker’, we discovered that Mfd translocates autonomously on DNA and efficiently localizes to a paused/stalled RNAP using a ‘release and catch‐up’ mechanism. This work demonstrates a remarkably delicate coordination between Mfd and RNAP, allowing efficient targeting and recycling of limited Mfd while providing expedient conflict resolution (Le et al, Cell, 2018). |
 Helicase HelicaseIn replication, to ensure accuracy, a replisome must effectively overcome numerous obstacles on its DNA substrate, including DNA lesions. We previously demonstrated that T7 helicase interacts strongly with a non‐replicating DNAP, forming a complex across the fork junction that permits the non‐replicating DNAP to travel alongside the unwinding helicase. (Sun et al, Nature Communications, 2015). We then found that T7 helicase, in association with non‐replicating DNAP, is able to displace a co‐directional RNAP and the DNAP can re‐initiate DNA synthesis using the RNA transcript (Sun et al, Nature Communications, 2018). These findings reveal a novel pathway of replication re‐initiation enabled by the participation of a replicative helicase and also broaden the long‐established role of replicative helicases. |
